KEY POINTS
- Determining the phase of hepatitis B virus (HBV) infection is essential to the clinical assessment of the patient with HBV.
- HBV DNA, HBeAg status and liver function testing are all vital components of this assessment.
- HBV DNA is an important parameter in informing treatment decision. Testing is Medicare rebatable for one test annually for monitoring and up to four tests annually for those on treatment.
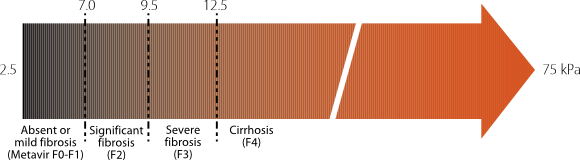
- Fibrosis assessment to determine the stage of liver disease is also important (non-invasive tests, imaging with or without biopsy)
- Non-invasive methods of assessing hepatic fibrosis such as transient elastography (FibroScan®) are becoming available.
- Normal alanine aminotransferase (ALT) ranges are being revised downwards (< 19 U/L for females and < 30 U/L for males), and normal liver function tests (LFTs) do not rule out significant hepatic disease.
- Transmission risks, lifestyle modification, cultural factors and long-term complications associated with chronic hepatitis B (CHB) infection are important components of patient education.
- When people are diagnosed with hepatitis B, testing, assessment and vaccination should be offered to their household and sexual contacts.
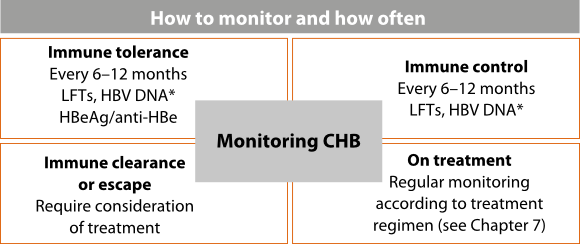
- All patients with CHB require regular monitoring for liver damage and disease progression.
Print as PDF
- Terrault N, Bzowej N, Chang K, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-83.
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98.
- Yi Z, Jie YW, Nan Z. The efficacy of anti-viral therapy on hepatitis B virus-associated glomerulonephritis: A systematic review and meta-analysis. Ann Hepatol. 2011;10:165–73.
- Digestive Health Foundation. Australian and New Zealand Chronic hepatitis B (CHB) recommendations. Summary and algorithm. 2nd edition 2010.
- Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Alimentary Pharmacol Ther 2008;28:1067–77.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9.
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996;24:289–93.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997;349:825–32.
- Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One 2012;7:e44930.
- Li Y, Huang Z, Wang Z, et al. Systematic review with meta-analysis: the diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Alimentary Pharmacol Ther 2016;43:458-69.
- Singh S, Muir A, Dieterich D, Falck-Ytter Y. American Gastroenterological Association Institute Technical Review on the role of elastography in chronic liver diseases. Gastroenterology 2017;152:1544–77.
- Kemp W, Levy M, Weltman M, Lubel J; Australian Liver Association (ALA). Australian Liver Association (ALA) expert consensus recommendations for the use of transient elastography in chronic viral hepatitis. J Gastroenterol Hepatol 2015;30:453-62.
- National Institute for Health and Care Excellence (NICE). Hepatitis B (chronic): diagnosis and management. Clinical guideline. Published 26 June 2013. Available at: nice.org.uk/guidance/cg165 (last accessed 23 June 2018).
- Sarin S, Kumar M, Lau G, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatology Int 2016;10:1-98.
- Hepatitis C Virus Infection Consensus Statement Working Group. Australian recommendations for the management of hepatitis C virus infection: a consensus statement. August 2017. Melbourne: Gastroenterological Society of Australia; 2017.
- Parikh P, Ryan JD, Tsochatzis EA. Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Ann Transl Med 2017;5:40.
- World Health Organization (WHO).Guidelines on hepatitis B and C testing. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
- Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835-47.
- Graham S, Guy R, Cowie B, Wand HC, Donovan B, Akre SP, et al. Chronic hepatitis B prevalence among Aboriginal and Torres Strait Islander Australians since universal vaccination: a systematic review and meta-analysis. BMC Infect Dis 2013;13:403.
- National Health and Medical Research Council. Australian guidelines to reduce health risks from drinking alcohol. Last updated 20 March 2018. Available at: https://www.nhmrc.gov.au/health-topics/alcohol-guidelines (last accessed 26 June 2018).
- Wong GL, Chan HL, Tse YK, Chan HY, Tse CH, Lo AO, et al. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology 2014;59:986–95.
- McMahon BJ. Epidemiology and natural history of hepatitis B. Sem Liver Dis 2005;25:3–8.