Laboratories
Laboratories that perform hepatitis C testing:
- must be NATA accredited for medical testing88
- must have a comprehensive quality management system, including standard operating procedures, documented training and competency assessment, internal quality control, incident reporting and result escalation pathways
- must participate in an external quality assessment scheme (EQAS)89
- must comply with the National Pathology Accreditation Advisory Council (NPAAC) standards.27
Point-of-care testing sites
Sites that perform hepatitis C testing:
- must participate in an external quality assessment scheme (EQAS)90
- should assess the competence of operators before testing and periodically consider accreditation under the National General Practice Accreditation Scheme, if appropriate90
- should perform regular quality control, monitor quantitative results graphically, have a validated method for establishing quality control acceptance limits and a documented procedure for managing result outside the acceptance criteria
- should comply with the Royal Australian College of General Practitioners Standards for point-of-care testing.91
The TGA regulates in-vitro diagnostic devices under the Therapeutic Goods Act 1989 (the Act) and its associated regulations.92 All commercially supplied hepatitis C in-vitro diagnostic devices must be included in the ARTG. Before being included in the ARTG, these in-vitro diagnostic devices are evaluated to ensure they are safe and perform as intended. Commercial kits labelled as Research Use Only (RUO), or approved in-vitro diagnostic devices used off label (not as described in the instructions for use), must be fully validated by the user before they can be approved by the TGA. Evidence of this validation must be submitted to the TGA for the test to be included as an in-house in-vitro diagnostic device in the ARTG.
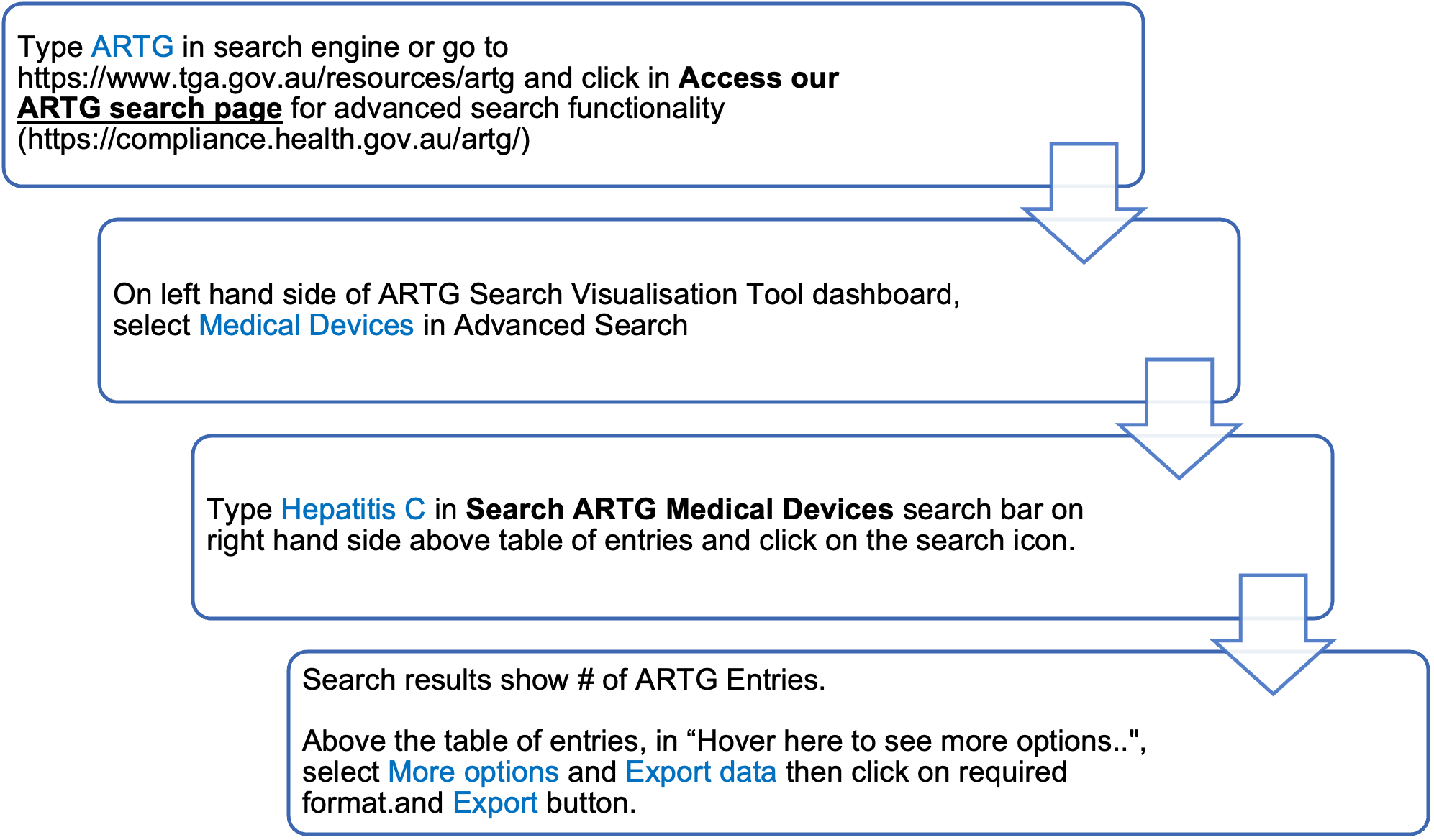
Laboratory users of commercially available hepatitis C assays can determine which are currently registered for use in Australia by searching the publicly accessible ARTG at https://www.tga.gov.au/resources/artg
To obtain a complete list of all available assays enter ‘hepatitis C’ into the ‘Search for’ field. Avoid using the abbreviation HCV, as only partial lists will be displayed. To find a particular assay, you may search by either the sponsor or assay name. Users of commercially available assays should seek advice from the sponsors of these kits to determine the purpose for which the assay is included in the ARTG.
Alternatively, https://compliance.health.gov.au/artg/ may be used for advanced search functionality.
To obtain a complete list of hepatitis C assays, follow the ARTG search flowchart below:
In-vitro diagnostic device manufacturers and sponsors in Australia have ongoing mandatory post-market monitoring responsibilities. Due to the high public health risk associated with hepatitis C in-vitro diagnostic devices, the TGA may impose specific post-market surveillance requirements as a condition of their inclusion in the ARTG. For instance, sponsors of point-of-care hepatitis C in-vitro diagnostic devices included in the ARTG must ensure that organisations using their in-vitro diagnostic devices participate in a hepatitis C point-of-care quality assurance program. These requirements can be found by searching for the specific hepatitis C in-vitro diagnostic device in the ARTG.
To comply with TGA legislation, sponsors must adhere to all imposed post-market surveillance requirements and report any known adverse events to the TGA. Manufacturers are responsible for investigating, implementing appropriate risk management procedures, and taking corrective and preventive actions related to their in-vitro diagnostic devices. If there are issues or deficiencies in safety, quality, or performance of in-vitro diagnostic devices already on the market, sponsors must initiate corrective actions in consultation with the TGA Recalls Section. This action must be undertaken as soon as practicable after becoming aware of any adverse events, malfunctions or deterioration in the performance, or inadequacies in the design, production or labelling of an in-vitro diagnostic device.
Users are encouraged to report any issues with an in-vitro diagnostic device to the sponsor and the TGA. Reports to the TGA can be submitted through the TGA’s Incident Reporting Investigation Scheme (IRIS) at: https://www.tga.gov.au/resources/resource/guidance/medical-device-incident-reporting-investigation-scheme-iris.
